Research
Research Policy
The basic direction of our research is “Elucidation of molecular and cellular pathogenesis of neuropsychiatric disorders and research on intervention methods. The main target diseases are Alzheimer's disease, autism, and Parkinson's disease. However, the following themes are not completely independent of each other, but are interrelated in terms of the development of treatments and interventions for these diseases. Also, new discoveries can be made by looking at things from the eyes of others that might not be noticed from each of our perspectives. In this regard, we believe that the lab members should always be “open minded, data sharing” with each other.
If you would like to receive PDF files of the English review articles listed in each section, please contact Prof. Tomita by e-mail.
Aβ Metabolic Mechanisms (Production, Secretion, and Degradation) and Their Regulation
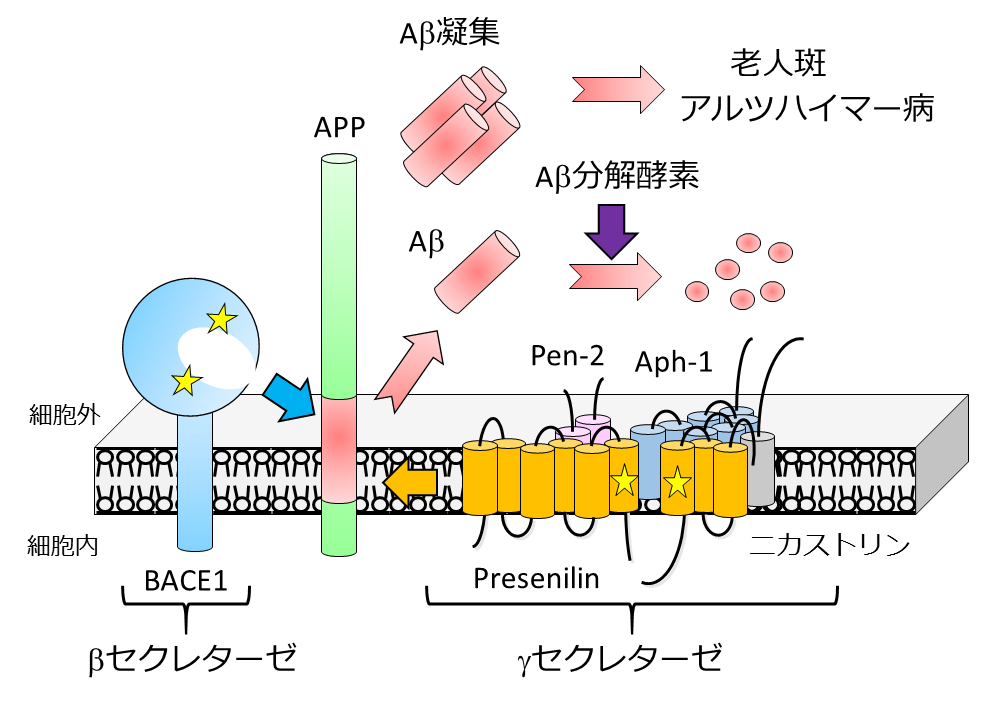
Aβ, a major component of senile plaques, is secreted when its precursor protein APP is cleaved by β- and γ-secretases. It has been shown that abnormalities in its production and degradation mechanisms are directly related to the risk of developing Alzheimer's disease.
We have focused on γ-secretase and developed methods to regulate its activity, and at the same time, we have established a new definition of protease as an “intramembrane sequence-cleaving enzyme. We also found that astrocytes release KLK7, an Aβ-degrading enzyme, to regulate the amount of Aβ in the brain. Thus, we believe that the full understanding of the mechanism of Aβ metabolism and the elucidation of its regulatory mechanism will lead to the development of therapeutic and diagnostic agents for Alzheimer's disease.
For a review article on the mechanism of cleavage of intramembrane sequences by γ-secretase, click here.
For a review article on proteases associated with APP and Aβ production, click here.
For a review article on intracellular vesicle trafficking and Aβ production, click here.
Understanding Cellular Pathogenesis Following Aβ Accumulation and Developing Diagnostic Methods
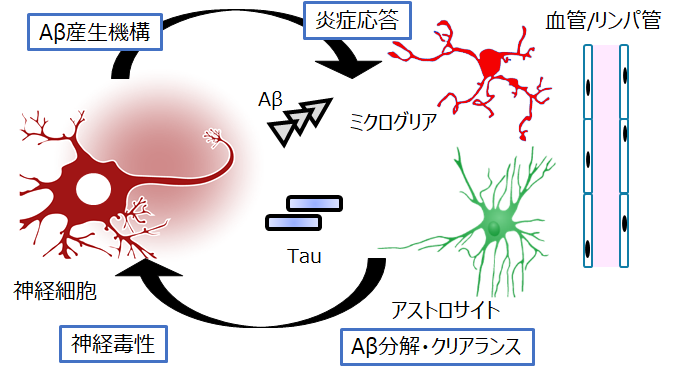
Results of large-scale observational studies have shown that Aβ accumulation occurs more than 10-20 years before the onset of Alzheimer's disease. Therefore, there is a need for a method to detect Aβ accumulation in the brain before the onset of the disease and to estimate the risk of developing Alzheimer's disease in the future.
It is now clear that the chronic changes that occur in the brain after Aβ accumulation affect not only neurons but also various cells, including glial cells and the vasculature. We are therefore aiming to elucidate the whole picture of cellular pathology, which refers to the response of individual cells and the changes that occur with chronicity after the accumulation of these aggregation proteins. We also aim to elucidate the production mechanism of recently identified blood biomarkers.
For a review article on astrocytes and amyloid accumulation pathology, click here.
Elucidation and Control of Amyloid Formation Mechanisms Accumulating in Patient Brains
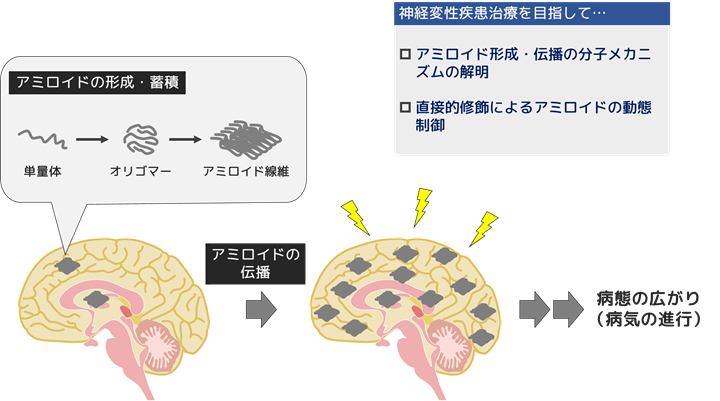
A characteristic pathology common to many neurodegenerative diseases such as Alzheimer's disease, frontotemporal dementia, and Parkinson's disease is the formation and accumulation of fibrils called “amyloids,” which are abnormal fibrous structures of proteins. Although the proteins that form amyloid vary widely, and the sites of formation and accumulation of amyloid vary from extracellular/intracellular to neuronal/glial cells, familial disease studies suggest that the formation of amyloid is the cause of disease onset in all cases. Therefore, controlling amyloid formation and its spread is considered to be an important therapeutic strategy for neurodegenerative diseases.
Our research aims to elucidate the mechanisms of amyloid formation and the spread of amyloid accumulation pathology (cell-to-cell transmission mechanism) at the molecular level and to clarify the mechanisms of pathogenesis. Furthermore, in collaboration with the Synthetic Organic Chemistry Laboratory, we are developing research on the dynamic control of amyloid by direct oxygen modification, with the aim of developing innovative therapeutic methods for neurodegenerative diseases.
For a review article on photo-oxygenation of amyloid β, click here.
For a review article on photo-oxygenation catalysts, click here.
Elucidating the Pathological Function of Microglia in Alzheimer's Disease
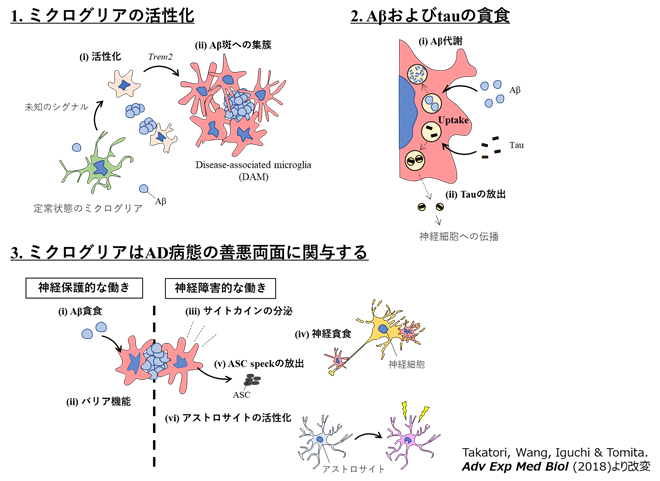
In recent years, genetic polymorphisms associated with the risk of developing Alzheimer's disease have been found in several genes specifically expressed in microglia, including TREM2. Microglia have also been implicated in the pathogenesis of many neuropsychiatric disorders such as autism spectrum disorders. Therefore, microglia may be effective drug target cells for multiple diseases.
On the other hand, the mechanism by which microglia, which are immune-responsive cells in the brain, respond to changes in the surrounding environment remains largely unknown. We are conducting in vivo analysis using mouse models of the disease and applying various imaging techniques such as optical and electron microscopy to investigate the mechanism of microglial information processing through research on the disease.
For a review article on Alzheimer's disease risk factors, click here.
Reproduction of Alzheimer's disease tau pathology
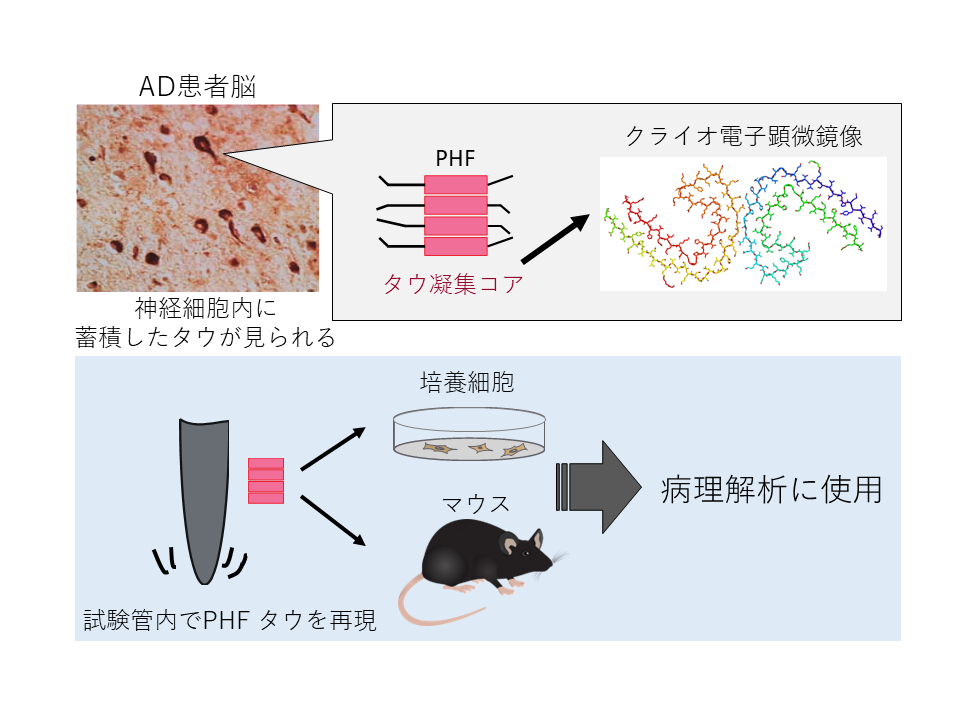
In order to elucidate Alzheimer's disease, it is desirable to use animal models that closely resemble human pathology. Recent technological innovations in clao-electron microscopy analysis have elucidated the structure of abnormal proteins that accumulate in patient brains. It is now clear that structural polymorphisms of tau exist in each disease, and that tau fibers formed in vitro have a different structure from those accumulated in the patient's brain. Therefore, it is assumed that it is necessary to reproduce the tau fibers that accumulate in the brains of patients with tauopathies.
We are therefore attempting to recapitulate the neurofibrillary tangles (PHF) in cultured cells and mice based on the structural information of tau proteins that accumulate in neurons. Through these studies, we aim to establish a model that can be used for drug discovery research by recapitulating human pathology, and at the same time, to elucidate the mechanisms that determine the specificity of different tauopathies.
For a review article on mouse models of Alzheimer's disease, click here.
Elucidation of Molecular Pathological Mechanisms of Parkinson's Disease
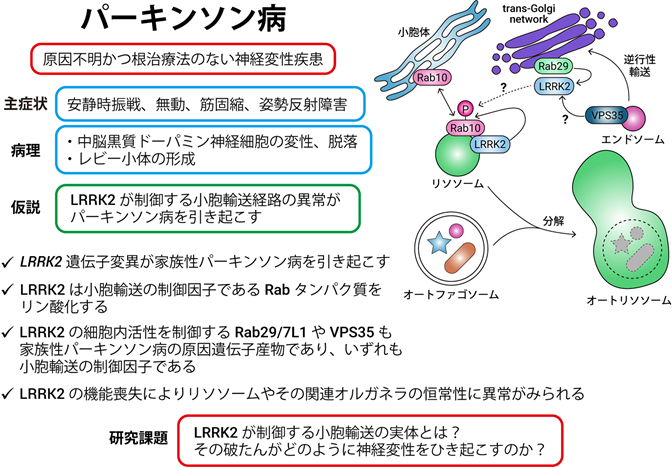
Recently, it has become clear that various genetic mutations cause familial Parkinson's disease, and among them, we are focusing on a kinase called LRRK2. LRRK2 phosphorylates several proteins belonging to the Rab family of vesicular trafficking regulators in vivo, and several vesicular trafficking-related molecules have been identified as having Parkinson's disease-causing genetic mutations.
Our research focuses on the physiological and pathological functions of Rab phosphorylation by LRRK2 and its regulation mechanisms by biochemical and molecular cell biological methods, aiming to elucidate the position of vesicular transport abnormalities in the molecular pathogenesis of Parkinson's disease and to develop innovative drugs for Parkinson's disease and diagnostics based on molecular mechanisms. We are also conducting research aimed at developing breakthrough Parkinson's disease therapeutics and diagnostics based on the molecular mechanism of the disease.
For a review article on LRRK2 and Parkinson's disease, click here.
Elucidation of the Pathogenic Propagation Mechanism of α-Synuclein Accumulation
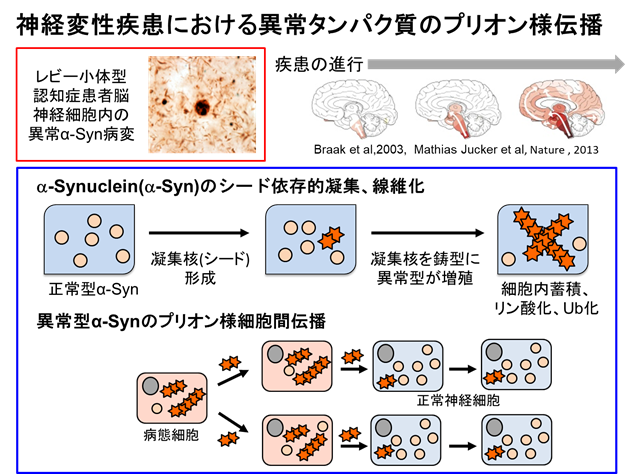
It has been shown that abnormal protein aggregates characteristic of neurodegenerative diseases have prion-like properties and may spread into the brain by cell-to-cell transmission. In particular, it has been demonstrated in vitro, in cultured cells, and in animal models that recombinant α-synuclein fibers and abnormal α-synuclein derived from the brains of patients with Parkinson's disease and multiple system atrophy can form aggregation nuclei (seeds) and cause prion-like spread.
To elucidate the pathogenesis and progression mechanisms of these diseases, we are studying the mechanisms of aggregate formation and propagation and the prion-like properties of aberrant protein aggregates accumulated in patient brains using currently established experimental models of prion-like propagation.
Based on what we have learned from our studies of Alzheimer's disease and secretase, we believe that abnormal membrane protein metabolism causes disease, and that we can develop methods to control the metabolic system.
Development of Dementia Prevention Exercise Programs
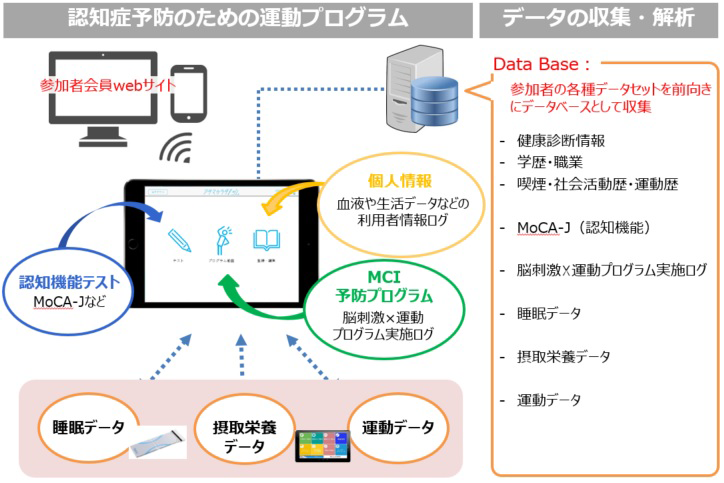
Considering the issue of healthcare costs and intervening in the early stages of dementia, there is a strong need to develop methods of dementia prevention other than drugs. In recent years, there have been a series of reports indicating that exercise may lead to the prevention of cognitive decline and dementia. Therefore, we are working with several companies to jointly develop exercise programs that contribute to the prevention of dementia.
In parallel with the implementation of the developed program, we will accumulate and analyze information on cognitive function and other biochemical parameters that may be related to the onset of dementia, as well as information on lifestyle habits such as diet and sleep. The goal is to obtain knowledge that will lead to the improvement of the accuracy of the program, which will contribute to essential disease prevention.
For a newspaper article on this exercise program, click here.
page top

